 |
|
|
|
|||||||||
|
 |
There is a direct relationship between growth in sales and market scandals in many of China's boom industries.
The health products sector is no exception.
One of the common problems is that most companies pay more attention to advertising the alleged benefits of their wares than actually ensuring that they provide quality products and services.
Even worse, there are few regulations and standards in place to ensure that the industry's advertising is fair and accurate, and the implementation of the standards that do exist leaves a lot to be desired.
In addition, a lack of trust hangs over the sector, trust that is further diminished by scandals over fake and shoddy products and deceitful publicity.
For example, the Beijing Times reported last August that a rural couple had been detained by police in Beijing for selling "fake Amway products".
More than 20,000 units of fake products were seized by the police, although it was not reported what they were meant to be.
Exaggerated publicity and fake products are key problems hindering the sustainable development of China's health products industry, according to Liu Pei, director-general of the policies and regulations department of the State Food and Drug Administration.
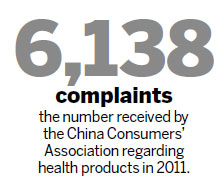
In 2011, the China Consumers' Association reported it received 6,138 complaints regarding health products, of which 2,611 were about quality, 812 dealt with exaggerated advertising, and 535 about fake products.
A further complication is that the country's health products supervision and administration regulations, which have been on the drawing board since 2008, have yet to be enacted.
Several versions of the draft law have been distributed for experts' discussion. But there is no telling when the final version will be ready for legislative approval.
Liu said that the law was being delayed because various authorities had failed to reach a consensus on the definition of health products.
The planned law would define the division of responsibilities among different government agencies, and how they should coordinate with each other, she said.
Apart from the State Food and Drug Administration, a number of other government agencies are involved in the supervision and administration of health products, including the ministries of health and commerce, the State Administration for Industry and Commerce, and the General Administration of Customs.