Robotic surgery may not be panacea after all
The biggest thing in operating rooms these days in the United States is a million-dollar, multi-armed robot named da Vinci, used in nearly 400,000 surgeries nationwide last year - triple the number of just four years ago.
But now the high-tech helper is under scrutiny over reports of problems, including several deaths that may be linked with it and the high cost of using the robotic system.
There also have been a few disturbing, freak incidents: a robotic hand that wouldn't let go of tissue grasped during surgery and a robotic arm hitting a patient in the face as she lay on the operating table.
 |
|
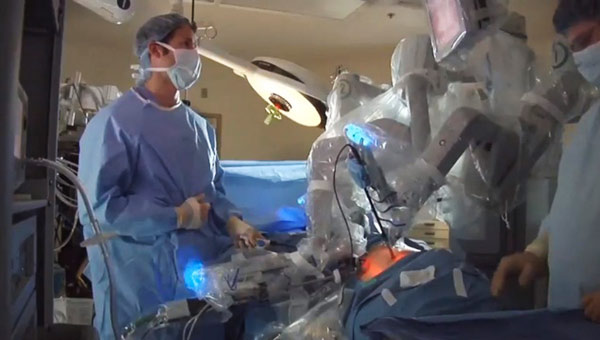
Robotic surgery equipment, such as the da Vinci shown here, has been hailed for its pinpoint accuracy, but US authorities are reviewing its benefits after reports of mishaps. Provided by Associated Press |
Is it time to curb the robot enthusiasm?
Some doctors say yes, concerned that the "wow" factor and heavy marketing have boosted use. They argue that there is not enough research showing that robotic surgery is at least as good or better than conventional surgeries.
Many US hospitals promote robotic surgery in patient brochures, online and even on highway billboards. Their aim is partly to attract business that helps pay for the costly robot.
The da Vinci is used for operations that include removing prostates, gallbladders and wombs, repairing heart valves, shrinking stomachs and transplanting organs. Its use has increased worldwide, but the system is most popular in the United States.
"We are at the tip of the iceberg. What we thought was impossible 10 years ago is now commonplace," said Dr Michael Stifelman, robotic surgery chief at New York University's Langone Medical Center.
For surgeons, who control the robot while sitting at a computer screen rather than standing over the patient, these operations can be less tiring. Plus robot hands don't shake. Advocates say patients sometimes have less bleeding and often are sent home sooner than with conventional laparoscopic surgeries and operations involving large incisions.
But the US Food and Drug Administration is looking into a spike in reported problems during robotic surgeries.
Earlier this year, the FDA began a survey of surgeons using the robotic system. The agency conducts such surveys of devices routinely, but FDA spokeswoman Synim Rivers said the reason for it now "is the increase in number of reports received" about da Vinci.
Reports filed since early last year include at least five deaths.
Last year there were 367,000 robot surgeries versus 114,000 in 2008, according to da Vinci's maker, Intuitive Surgical Inc of Sunnyvale, California.
Da Vinci is the company's only product, and it's the only robotic system cleared for soft-tissue surgery by the FDA. There are other robotic devices approved for neurosurgery and orthopedics, among other things.
The da Vinci system "has an excellent safety record with over 1.5 million surgeries performed globally, and total adverse event rates have remained low and in line with historical trends," said company spokeswoman Angela Wonson.
But an upcoming research paper suggests that problems linked with robotic surgery are underreported. They include cases with "catastrophic complications", said Dr Martin Makary, a Johns Hopkins surgeon who co-authored the paper.
"The rapid adoption of robotic surgery ... has been done by and large without the proper evaluation," Makary said.