Pharma and medical exports likely to surge
Updated: 2012-01-18 11:25
By Liu Jie (China Daily)
|
|||||||||
|
Health products on sale at a supermarket in Shanghai. China's exports of medicines and health products surged in the first 11 months of last year. [Photo/China Daily] |
Chinese firms look to emerging economies to grow market share
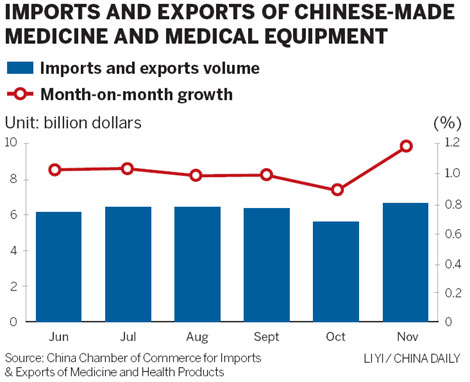
BEIJING - The growth of imports and exports of Chinese-made medicines and health products almost doubled the growth rate of the nation's total foreign trading volume as a whole during the first 11 months of last year, and is expected to continue increasing quickly this year.
Meanwhile, Chinese exports of pharmaceuticals and medical equipment to emerging markets such as India, Brazil and Russia, witnessed a dramatic surge over the same period. The increase implies that Chinese enterprises are diversifying their export destinations to avoid trading barriers in developed markets, according to the China Chamber of Commerce for Imports & Exports of Medicine and Health Products on Tuesday.
Meng Dongping, the chamber's vice-chairwomen, said that the industry's foreign trade will continue to grow during the coming years, given the rapid development of Chinese companies through business upgrading, greater international cooperation and the ever-increasing demand in the Chinese market.
The chamber forecast that Chinese exports of pharmaceuticals and medical equipment will register a year-on-year increase of 20 percent in 2012. Growth in the emerging markets is expected to be higher.
Statistics from the chamber, a State-owned industry association under the Ministry of Commerce, show that the trading volume of China's pharmaceuticals and medical equipment sector totaled $66.02 billion between January and November 2011, with a year-on-year increase of 39.4 percent, compared with 23.6 percent growth in the nation's total imports and exports over the same period.
China's exports of medicines and health products to the traditional markets, including the United States, Japan and Germany, rose by $2.8 billion in the first 11 months of last year from 2010, $700 million lower than in emerging markets.
At the same time, exports to India rose by 28.24 percent year-on-year, while those to Brazil increased by 48.69 percent. Russia saw growth of 47.27 percent and South Africa registered an increase of 31.39 percent.
"Africa will also be a promising market for Chinese exporters in the near future," said Xu Ming, an official from the chamber."So far, traditional markets still account for a large proportion of China's exports. Chinese companies have advantages in generic medicines and the middle- and low-end medical-device sectors."
Despite the rapid growth, China's trade in the sector faces a number of uncertainties this year, including gloomy prospects for the international market, fluctuations in the global financial market and a number of trading barriers, said Meng in an earlier interview.
In May 2011, the European Union (EU) imposed new regulations on the registration of makers of traditional Chinese medicines, hindering the import of proprietary Chinese medicines. The EU also issued a regulation in June that raised the import threshold of medicines and active pharmaceutical ingredients, which are important Chinese exports to the EU. Emerging markets, such as Peru and Brazil, also increased their rules on drug quality and registration.
"Those regulations pose challenges to Chinese exporters. We are making efforts to help Chinese companies to improve the quality of their products and to become familiar with international guidelines. We are also negotiating with relevant foreign authorities and industry associations to lower trading barriers," said Meng.
An injected cancer treatment made by Jiangsu Hengrui Medicine Co Ltd won approval from the US Food and Drug Administration (FDA) on Dec 16, making Hengrui the first Chinese pharmaceutical company to obtain a license to sell the treatment in the US.
A report by Shenyin & Wanguo Securities Co Ltd said the approval demonstrates Hengrui's strength, as well as the difficulties Chinese drugmakers face when entering foreign markets. Jiangsu-based Hengrui has been aiming to enter the international market for several years and first applied to the FDA for a license to sell the cancer injection in 2008.
"It's increasingly difficult for Chinese enterprises to enter developed markets," said a manager of Hengrui, who spoke under condition of anonymity. "We are facing the problem of how to establish our sales network in the US to make profits."
Hengrui now is applying for licenses to sell more of its generic medicines in the US and EU.
Related Stories
Medical product export up nearly 30% 2006-02-06 15:41
S. Korea to allow exporting narcotics for medical treatment 2009-12-29 14:26
Neusoft produces medical equipment for export 2003-07-21 10:18
- Grain crop heads for ninth year of increase
- Businesses to benefit from festival
- China to help drive world GDP expansion
- PBOC injects cash into banks via reverse repo
- Farm produce prices rise for 8th straight week
- China's FDI falls 12.73% in Dec
- China's 2012 home price to drop by 5.3%
- Urbanization to drive consumption