Stemming the flow
By Todd Balazovic | China Daily | Updated: 2009-12-24 07:47
|
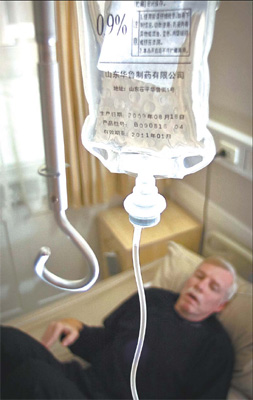
Richard Jewell, 54, an American sufferer of Parkinson's disease who has paid more than $30,000 for stem cell treatment, waits in his room in Tiantan Puhua Hospital in Beijing.Photos by Jonah M. Kessel |
China plans to tighten laws to halt stem cell treatments as questions on effectiveness and safety remain, reports Todd Balazovic
Photo