Chinese pharma companies look West
Updated: 2013-05-20 07:38first sold in the United States, then expanded into Europe and South America," said Xie Liangzhi, president and CEO of Sino Biological, adding that he believes leveraging the multinational's platform to brand his young business in developed markets will be efficient and cost-saving.
"Business partnerships are like a marriage," said John Lechleiter, chairman, president and CEO of Ely Lilly & Co, explaining that the partners must have common goals and philosophies as well as mutual respect and mutual admiration. An adaptation period is also necessary.
At present, a large part of the collaborations between Chinese and foreign pharmaceutical companies are in the branded generics sector.
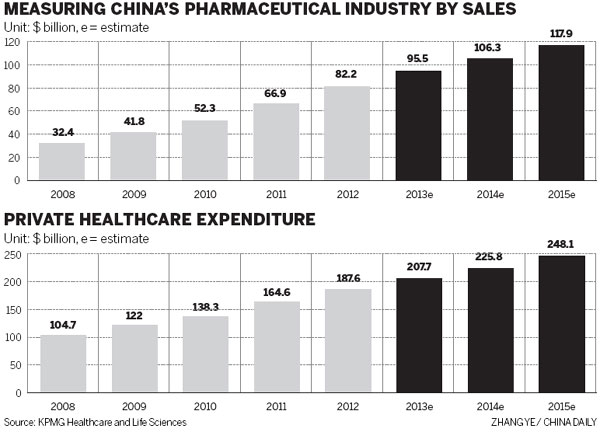
Since 2011, many innovative blockbuster drugs made by pharmaceutical giants have begun to come off-patent while the development of new ones has not been finalized. Insiders call it a patent cliff.
According to Datamonitor Plc, a UK-based market research company, there will be more than 30 blockbuster drugs due to go off-patent and lose their price protection between 2011 and 2016. Their sales were nearly $60 billion in 2011. That means the market is beginning to be flooded with generics, priced much lower than innovative medicines. Demand for these off-patent drugs will boom given their recently acquired affordability.
Norbert Meyring, head of KPMG Healthcare and Life Science in China and Asia-Pacific, said: "The patent cliff, faced mostly by multinationals, will lead to more acquisitions and joint ventures in China." Multinationals that mainly rely on patented drugs are striving to use high-quality branded generics to offset their losses in this patent cliff time so they are anxious to find the right Chinese manufacturers to expand production capacity, he added.
Chinese pharmaceutical companies have accumulated rich experience in the production of generic medicines.
Furthermore, production costs in China, although increasing in recent years, are comparatively low. The cost-performance is still lucrative for international giants, said Shi Lichen, a partner of domestic consultancy firm Alliance PKU Management Consultants Ltd.
He added that Chinese companies are gradually maturing. Depending on their manufacturing strength, the domestic leading players are confident of entry into the international generics market. Together with the enhancement of their R&D capabilities, they believe, through cooperation with international majors, the innovative sector will not be beyond their reach in the near future.
"Chinese partners should be clear-headed, avoiding becoming just manufacturing bases for multinational companies," said Shi. He urged Chinese companies to make full use of cooperation opportunities to learn management and research expertise from international majors instead of being devoted solely to production. "Market access just indicates short-term profits, whereas increasing soft strength is a long-term investment," Shi said.
Li Dongjiu, senior vice-president of Shanghai Fosun, said he believes the Sino-foreign cooperation, in any kind of model, is natural and both sides are profit-driven. "In the internationalized market, collaborations and exchanges over technology, capital, talent or information are natural and necessary," he said. "Chinese companies are growing. The international pharmaceutical market is huge with potential in various areas. We welcome both competition and collaboration at home and abroad."
Shanghai Fosun Pharmaceuticals (Group) Co is a pioneer of globalization. It is expanding its overseas business in part via small-sized mergers and acquisitions. In addition to the acquisition of Saladax Biomedical, Wanbang Biopharma Co, a subsidiary of Fosun Pharmaceutical, reached an agreement with German company Biotest AG at the end of last year in which Wanbang will sell Biotest's human serum albumin on the Chinese mainland. Human serum albumin is the most abundant protein in human blood plasma.
"We are introducing foreign products to China while leveraging their platform to deliver our products and technologies abroad," said Li, stressing that Fosun's overseas expansion should be based on domestic development, with the domestic market a priority before going abroad.
"We are eyeing fast-growing counterparts in the US, Europe and Japan for mergers and acquisitions. They should have strong R&D capabilities and be unique in terms of products, technology or distribution teams," he said.
Although Chinese companies have gradually won recognition in the medical industry in developed markets, they are little known by Western customers. Many Westerners are surprised to hear of the existence of Chinese medicines other traditional herbal remedies.
Derrick Board, 57, from England, said he was shocked to learn Chinese pharmaceutical companies exist. He said Chinese homemade herbal medicine and acupuncture were all he knew about Chinese treatments.
Describing his impression of Chinese medicine, he said "curious, interesting, alternative, historical and wholesome" are adjectives that immediately come to mind.
"I would be happy to use Chinese medicine and Chinese medical equipment if they gain European Union certifications," said Board.
John Edwards, 48, also from England, holds the same view as Board. He had never heard of, but was happy to try, Chinese pharmaceuticals. However, he said he would use the word "unethical" to describe his impression of Chinese medicine. "I think Chinese medicine has an annoying habit of using endangered and nearly extinct animals," Edwards said.
Zhang, of the China Pharmaceutical Technology Organization Expert Committee, said: "Getting the West to really understand Chinese medicines, particularly modern Chinese medicines, will be really tough work. Market access is the first step. Brand-building will take time."
- BYD exports three electric cars to Thailand
- Grid gets first jolt of residential solar power
- US now largest buyer of China's exports
- China's outbound M&As on the rise
- Tobacco control may entail price, tax rises
- Quanzhou becomes pilot financial reform zone
- New automobiles shine at Geneva Motor Show
- World's longest high-speed rail 'on track'
- Jiugui Liquor involved in plasticizer scandal again
- Accident reignites school bus safety concerns
- China to revise labor law
- Trademark registration under scrutiny
- Dinner ban takes toll on liquor firms
- CIC tables bid for London's Chiswick Park
- Property buyers eye overseas market
- Call for law to protect personal information
- China to cut train ticket prices
- Christmas business
- Solar industry to get jolt from new policies
- KFC chicken under spotlight















