China conditionally approves four self-developed COVID-19 vaccines: official
Share - WeChat

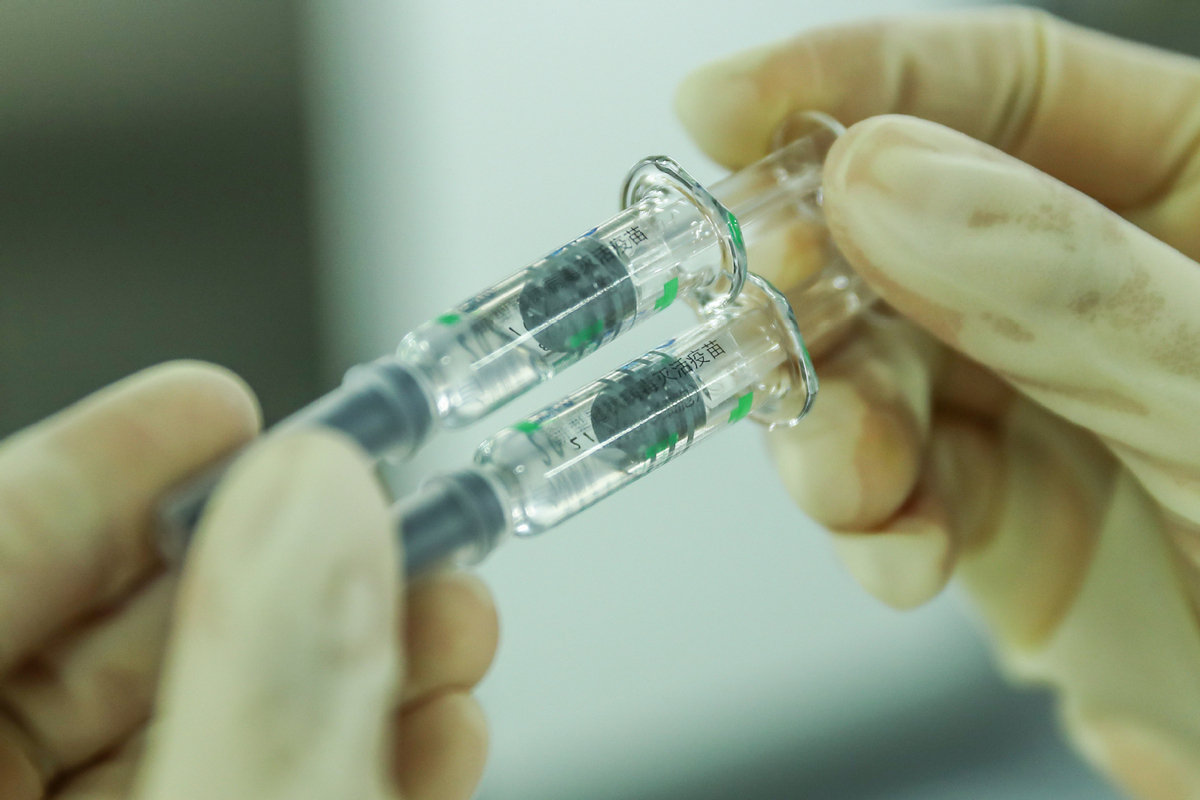
BEIJING - China has granted conditional market approval to four self-developed COVID-19 vaccines, Minister of Science and Technology Wang Zhigang said Friday.
China has adopted five technological approaches in developing COVID-19 vaccines. China now has 17 self-developed COVID-19 vaccines undergoing clinical trials, with seven undergoing phase-3 clinical trials, Wang told a press conference held in Beijing.
China has also included 11 drugs and treatment methods in its diagnosis and treatment plan for COVID-19, Wang added.
- Ten photos from across China: Jan 30 – Feb 5
- China health body supervises psychiatric fraud probe
- Bamboo-based drone completes maiden flight in Tianjin
- Hunan emerging as China-Africa trade hub
- Former senior Guizhou health official jailed for bribery
- Snowfall gives Gansu mountain town a breathtaking appearance




































