Sinovac applies for conditional marketing authorization of COVID-19 vaccine

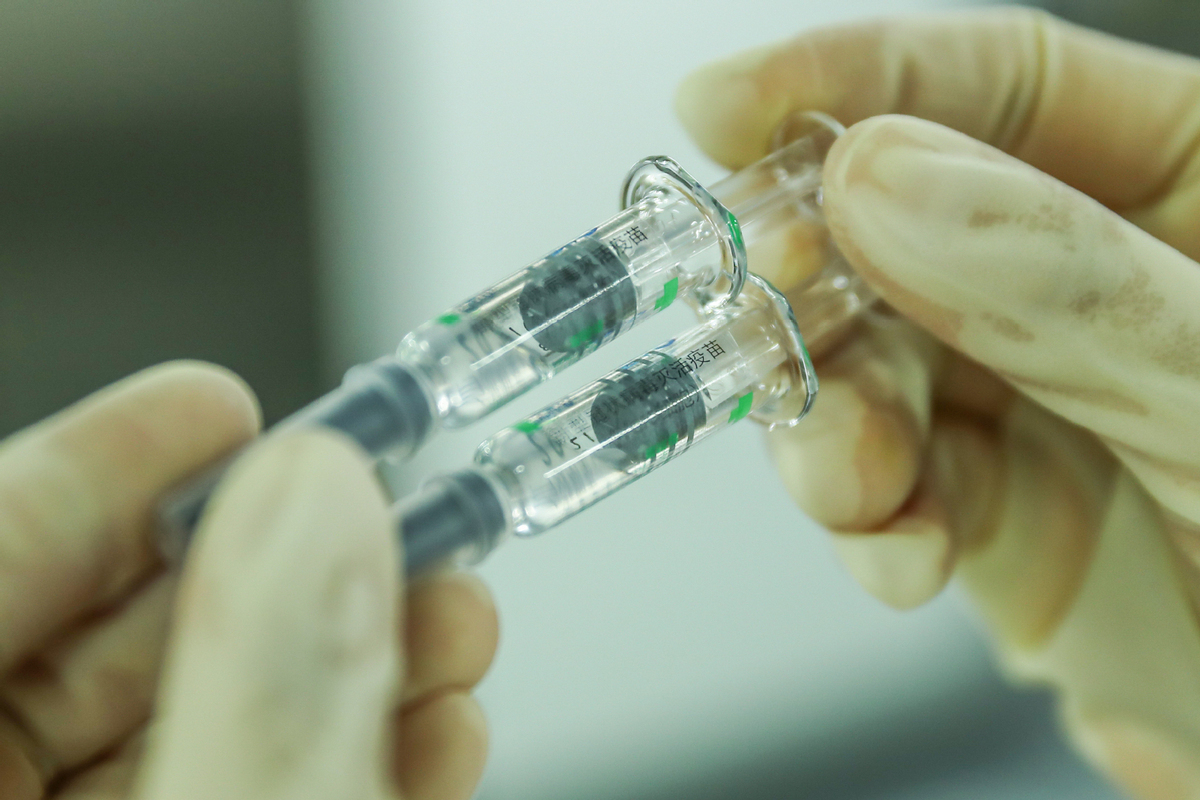
BEIJING -- Beijing-based Sinovac Life Sciences Co., Ltd. on Wednesday filed an application with Chinese authorities for conditional marketing authorization of its anti-COVID-19 vaccine CoronaVac.
The application has been received by the National Medical Products Administration (NMPA), according to Sinovac Biotech Ltd., which has a majority stake in the vaccine producer.
The inactivated vaccine CoronaVac has made progress in phase three clinical trials overseas, showing a good level of safety after inoculation, according to a company statement.
Fourteen days after the inoculation of two doses of CoronaVac, the effectiveness of the vaccine in protecting against diseases caused by COVID-19 has met the relevant technical standards laid out by the World Health Organization and the requirements of the NMPA, the statement said.
- Community memory clinics help aging minds stay connected
- Guangzhou's bald cypress trees turn red after cold snap
- Chinese researchers create novel computing architecture for major power boost
- Chinese astronauts conduct key training, experiments on space station
- China maintains strong momentum in anti-corruption drive
- China maps cotton's evolutionary secrets to build better crops




































