The huge potential of 'flammable ice' as a new source of energy
Updated: 2013-09-29 07:26
By Henry Fountain(The New York Times)
|
|||||||||
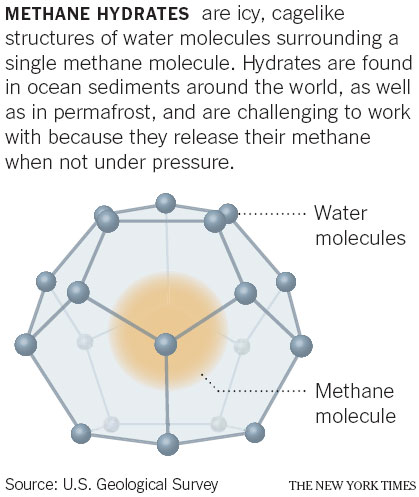
SAPPORO, Japan - The road to Japan's energy future may run through Sapporo, where researchers are studying sediment cores containing methane hydrates, icy constructs of water molecules with the explosive gas methane trapped within.
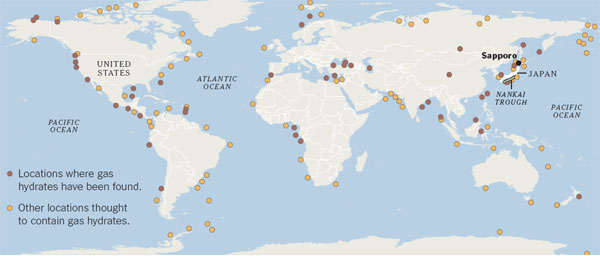
Hydrate-containing sediments are found in large amounts around the world, both under the sea and in permafrost. If they can be tapped safely and economically, they could be an abundant source of fuel, especially for countries like Japan that have few energy reserves of their own.
The Japanese researchers' work has already borne fruit: in March, the government said it had produced methane from hydrates in sediments under the Pacific Ocean. The effort, conducted from a drilling ship in the Nankai Trough about 160 kilometers east of Osaka, was the world's first hydrate production test in deep water.
But scientists say much is unknown about the compounds, sometimes referred to as "flammable ice." "We need to know more about the physical properties of hydrates themselves, and of the sediments as well," said Hideo Narita, head of the research lab, part of the National Institute of Advanced Industrial Science and Technology.

Further research will help scientists better understand the environmental impact of hydrate production, including the possible release of methane, a potent greenhouse gas. There is also the potential for subsea landforms to become unstable when hydrates are removed.
Timothy S. Collett, with the United States Geological Survey, said that despite all the talk about their potential as an energy resource, "hydrates are largely still a scientific issue."
The research poses special challenges because hydrates form under high pressure, caused by the weight of all the seawater or rock above them, and that pressure must be maintained when the sediment cores are analyzed. If it is not, the hydrates within quickly dissociate into water and gas, said Carlos Santamarina of Georgia Tech in Atlanta.
|
Hydrates are combinations of methane and water. J. Pinkston and L. Stern / Usgs |
Methane hydrates have bedeviled petroleum engineers for decades, as they can form in subsea pipelines and obstruct flows. They played a small but unwelcome role during efforts to stop the Gulf of Mexico oil spill in 2010, quickly clogging a huge steel box designed to funnel the oil safely to the surface.
Running into hydrates while drilling can also complicate exploitation of conventional oil and gas reserves. But for years, scientists have considered that hydrates might be an energy source in themselves.
Hydrates can sometimes appear as clumps of ice on the seafloor. However, for energy production, researchers are most interested in those that form in sediments. They are created when methane - which is produced by microbes, or heat and pressure, acting on organic matter - migrates upward in the sediments and mixes with water under specific conditions of temperature and pressure.
The icy substance forms in the microscopic spaces between the sediment grains, often in substantial amounts.
"You have a lot of methane, you have a lot of water, and guess what, they're going to form hydrates," said Carolyn Ruppel of the geological survey.
Sandy sediments, with bigger grains, are preferred over clay. "They're very permeable, so it's easy to get the hydrates out," Dr. Ruppel said.
Conventional techniques would not work well in clays, which contain the vast majority of known hydrate reserves, because the pore sizes are much smaller, Dr. Santamarina said. Outside-the-box thinking will be required to come up with ways to extract methane from them. "Much of the current paradigm for production in methane hydrates is anchored around oil production," he said. "And probably with that paradigm we may not go very far."
"We'll have to come up with smart solutions," he added. "It will take good engineering to figure it out," he said.
The New York Times
(China Daily 09/29/2013 page11)
