Federal judge in Texas could ban abortion pill

A federal judge heard arguments Wednesday from a coalition of anti-abortion groups seeking to overturn the Food and Drug Administration’s approval of an abortion medication that has been on the market in the US for more than two decades.
US District Judge Matthew Kacsmaryk held a four-hour hearing in Amarillo without issuing a ruling but promised that an order and an opinion would be issued “as soon as possible”.
The questions he asked both sides in the case suggested that he is contemplating to at least block the US government’s recent move to make the medication mifepristone more widely available or ban the drug entirely.
Kacsmaryk, who was appointed by Republican former president Donald Trump in 2018, appeared to be sympathizing with the legal arguments from challengers who argued the FDA’s approval was unlawful.
Kacsmaryk also had many questions for the plaintiffs and showed some skepticism toward granting the total ban on the drug that they seek, according to CNN.
The suit was filed against the Food and Drug Administration (FDA) in Amarillo by Alliance Defending Freedom, a group that has been involved in antiabortion litigation on behalf of four antiabortion medical organizations and four doctors. The suit also named the Health and Human Services Department as a defendant.
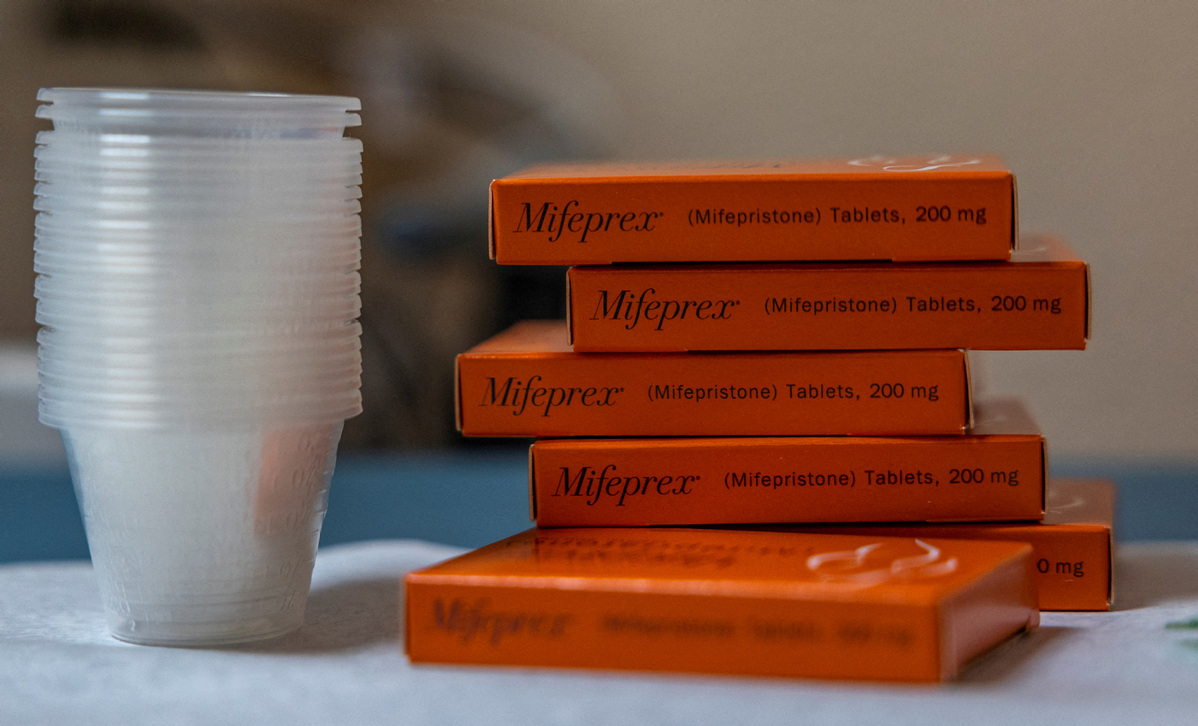
The plaintiffs claim that the FDA lacked the authority to approve the drug, didn’t adequately study the medication, and that the drug is unsafe. They asked Kacsmaryk to issue an immediate order that would revoke or suspend mifepristone’s approval, which the FDA approved in combination with a second pill as a safe and effective method for ending pregnancy in 2000.
Lawyers from the Justice Department who represented the FDA cited evidence that mifepristone was extremely safe and contended that the plaintiffs didn’t have any legal standing to file the lawsuit because none of them could show that the FDA approval had caused them harm.
Mifepristone, when combined with a second pill, has become the most common method of abortion in the US and has been increasingly prescribed since Roe vs Wade was overturned by the US Supreme Court in June 2022.
The FDA said that mifepristone also is widely prescribed for treatment of miscarriages and incomplete abortions. It is also used for treatment of uterine fibroids, endometriosis, reduce hemorrhaging during certain serious pregnancy complications.
Historically, the FDA’s authority to regulate prescription drugs hasn’t been challenged and there is no precedent for a judge to overrule the scientific decisions of the FDA. Some legal experts have warned of far-reaching consequences if judges begin second-guessing FDA decisions on drug safety and effectiveness, according to The Associated Press.
Kacsmaryk, who has worked as an attorney for a Christian legal group and voiced views against abortion, is the sole federal judge in his district. Any cases filed there will go to him.
As a result, conservatives have been filing cases seeking to roll back reproductive rights and LGBTQ rights or blocking policies of the Biden administration in Amarillo.
Kacsmaryk also has been criticized for a lack of transparency about the hearing, which became public only two days before the hearing. Typically, court hearings are scheduled weeks or months in advance, and the schedules are promptly added to the public docket.
He told lawyers Friday that he would delay the filing to minimize threats and possible protests and asked them not to disclose the date of the hearing, according to The Washington Post.
After news organizations learned of the session anyway and reported it on Sunday, the judge posted an announcement of the hearing on Monday.

































