Stunning drug revives hope for Alzheimer's treatment

Big hope
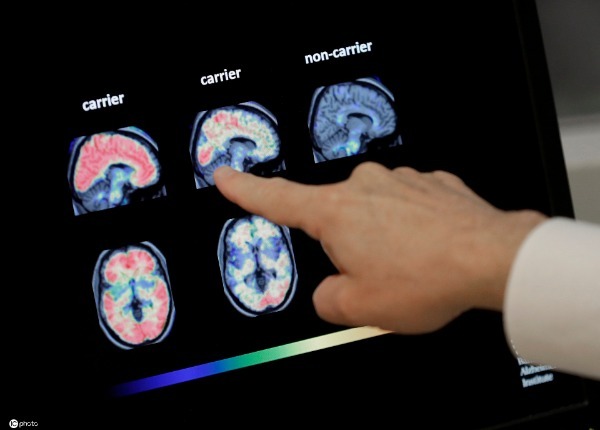
Alzheimer's disease is a common form of dementia, believed to be caused by changes in the brain usually beginning in late middle age. It is characterized by memory lapses, confusion, emotional instability and progressive loss of mental ability.
There haven't been any new dementia drugs for the past decade. Only six Alzheimer's disease drugs have been approved by the US Food and Drug Administration and nine drugs have been listed in other countries, according to a report from the Pharmaceutical Research and Manufacturers of America. Though improving cognitive and memory disorders, none could prevent or delay the progression of Alzheimer's.
This could be attributed to the high failure rate of clinical trials. A total of 146 Alzheimer's drugs worldwide have failed in clinical research and development centers between 1998 and 2017, around 18 percent of which failed in the late phase. Even pharmaceutical giant Pfizer announced the closure of new drug development for Alzheimer's treatment last January.
No hesitation
Despite the setbacks, some countries have spared no effort to conquer the disease, yielding impressive outcomes over the past decades.
China has about 10 million people with Alzheimer's, ranking first in the world. In July of 2018, Chinese pharmaceutical company Green Valley and other scientific research units jointly completed the clinical phase 3 trial of a new dementia drug, GV-971, which was based on a 22-year study and met expectations for cognitive improvement.
Last November, the State Drug Administration accepted the GV-971 listing application.
Alzheimer's disease may become commonplace as human life expectancy increases. Netizens believe if the development of medical care is temporarily unable to keep up with the pace of dementia, more companionship will make seniors feel less lonely.

































