Two workshops in Yizhuang obtains GMP certificate
( Etimes )
The Yizhuang Plant of Tiantan Biological Products announced that the OPV workshop and MMR workshop have received GMP certification, which guarantees the production of the company.
The certificates issued include: small-volume injection (sterile water for injection), poliomyelitis live attenuated vaccine sugar pill (human diploid cell), oral poliomyelitis live attenuated vaccine (human diploid cell), measles live attenuated vaccine, parotitis live attenuated vaccine, rubella live attenuated vaccine (human diploid cell), measles and rubella live attenuated vaccine, and MMR live attenuated vaccine. They are valid until August 21, 2019.
Tiantan Biological Products began moving to the Yizhuang base gradually since January this year. Yizhuang Vaccine Industry Base of Tiantan Biological Products was financed with 2.66 billion yuan in total and covers an area of about 160,000 square meters. It is the largest construction project in China's biological products industry, including nine production workshops, 15 auxiliary workshops and relevant supporting facilities workshops.
The building area is about 249,000 square meters. A total of 17 types of vaccines are ready to be produced, including the adsorption DPT vaccine, adsorption diphtheria vaccine, AC meningococcal vaccine, measles and rubella live attenuated vaccine, and MMR live attenuated vaccine.
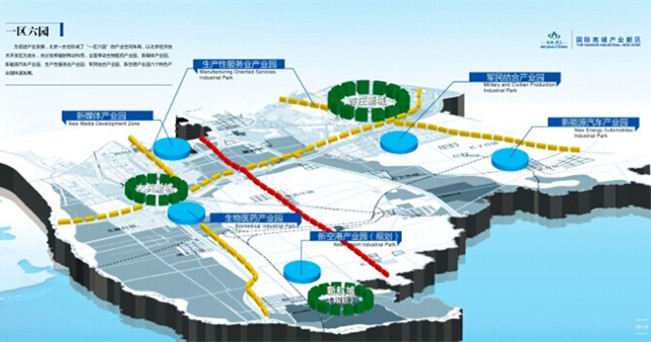 The Area with Six Parks
The Area with Six Parks Global Top 500
Global Top 500