|
Birth-control patch users face clot risk
(AP)
Updated: 2006-02-18 08:52
WASHINGTON - Risks of blood clots in legs and lungs are twice as high for
women using the birth-control patch instead of the pill, says a study reported
by the drug maker and the Food and Drug Administration.
Dr. Daniel Shames of the FDA said Friday the new findings don't require
immediate action by the government, but he urged concerned women to discuss the
risk with their physicians.
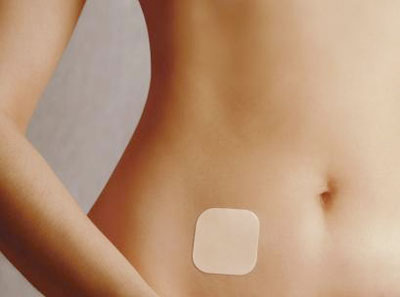
The world's first
contraceptive patch, made by Ortho-McNeil Pharmaceuticals, a subsidiary of
Johnson & Johnson, is shown in this undated photo. Women who use the
Ortho Evra birth-control patch face twice the risk of developing blood
clots than those who take the pill, the patch's manufacturer said, citing
recent company-funded research, Feb. 16, 2006.
[AP] | One new study found users of the Ortho Evra
patch had twice the risk of clots compared with women taking birth-control
pills, although a second analysis found no difference in risk between the two
forms of birth control.
"For some people the patch may be better because some people don't reliably
take the pill, or don't want to take the pill or forget the pill," Shames said.
"So the patch does offer them some alternative for contraception."
"On the other hand, we need to interpret what these results mean," he said.
"But these results are preliminary so we can't make hard comments about it."
The results of the two studies were made public Thursday by the patch's
manufacturer, Ortho Women's Health & Urology. The Raritan, N.J.-based
company is owned by Johnson & Johnson.
Last year an investigation by The Associated Press, citing federal death and
injury reports, found higher rates of blood clots in women using the patch.
While one of the newly reported studies found no increased risk of clots, the
interim results from the second suggested a twofold increase in the risk of
venous thromboembolic events, or clots in the legs and lungs, in women using the
patch, Ortho said.
However, because the confidence intervals of the results for the two forms of
contraceptive overlap, there actually may be no increased risk from the patch or
it may be more than double, said Shames, FDA's director for reproductive and
urologic drug products.
He said the risk of a nonfatal blood clot is about one per year in 10,000
women not using a contraceptive. For those using a hormonal contraceptive such
as the patch or pill the risk rises to between three and five, he said.
"These are fairly unusual events," said Shames. He noted that in preapproval
testing of the patch on about 3,000 women there were two reports of blood clots,
but one involved a woman who had had surgery.
The ongoing studies also are looking at the risk of heart attacks and strokes
among users of the two types of contraception. Currently there is no difference
but the numbers are small and it will take another 18 months to see if a
difference occurs, Shames said.
The company said the risk of clots remains rare and that they have been
reported as a potential risk of all hormonal contraceptives.
Release of the interim results comes four months after the FDA warned women
that the increased levels of hormones released by the patch put them at higher
risk of blood clots and other serious side effects.
Additions to the patch label made in November warned women that they would be
exposed to about 60 percent more estrogen than those who use birth-control
pills.
Since the patch went on sale in 2002, more than 4 million women have used it.
The investigation by The Associated Press found that patch users die and
suffer blood clots at a rate three times higher than women taking the pill.
About a dozen women died in 2004 from blood clots believed linked to use of the
patch, the AP reported. Dozens more suffered strokes and other clot-linked
problems.
Health officials warn that women who smoke should not use the patch, since
smoking increases the risk of stroke and heart attack.
|