China conditionally approves four self-developed COVID-19 vaccines: official
Share - WeChat

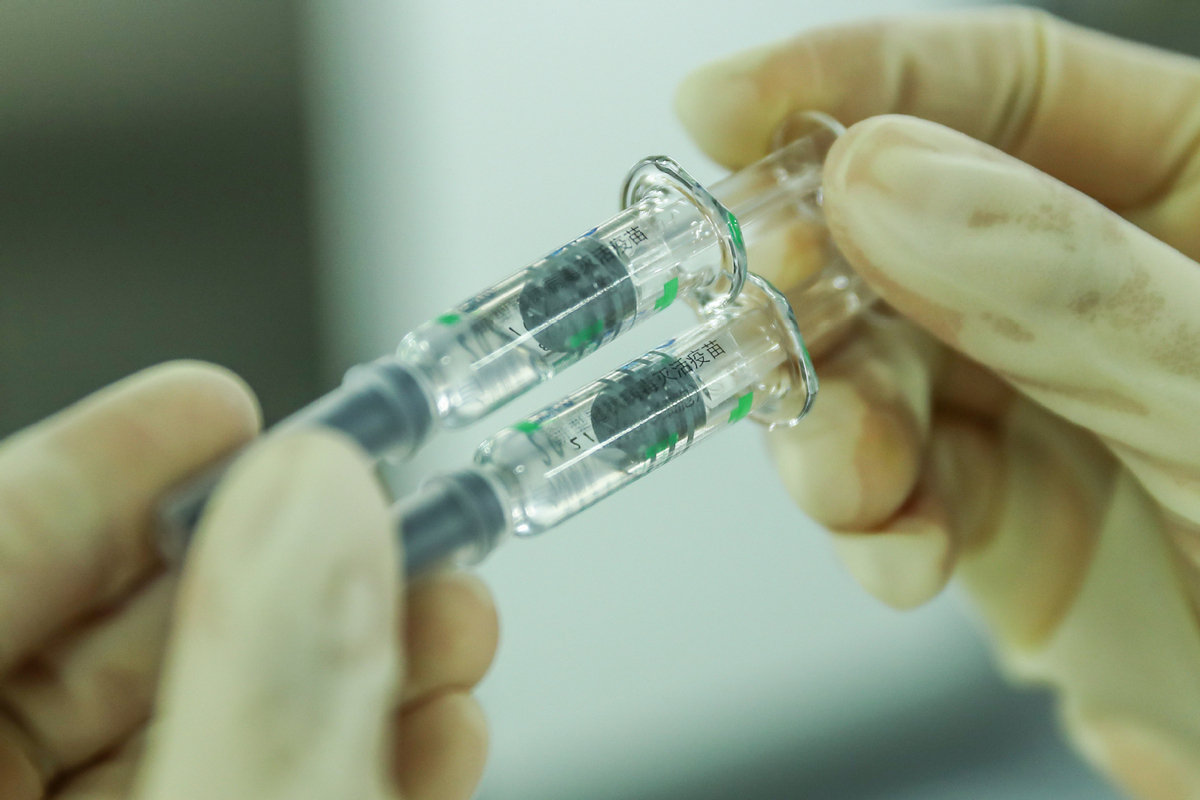
BEIJING - China has granted conditional market approval to four self-developed COVID-19 vaccines, Minister of Science and Technology Wang Zhigang said Friday.
China has adopted five technological approaches in developing COVID-19 vaccines. China now has 17 self-developed COVID-19 vaccines undergoing clinical trials, with seven undergoing phase-3 clinical trials, Wang told a press conference held in Beijing.
China has also included 11 drugs and treatment methods in its diagnosis and treatment plan for COVID-19, Wang added.
- One year on, life is beautiful in quake-hit Xizang
- Polished inbound tourism sector sparkles brightly again
- Ex-Gansu vice-governor convicted of bribery, insider trading
- Shenzhen's innovative companies display their latest tech and products to a global audience
- Beijing slams Tokyo's security documents revision plan
- China's central bank signals flexible policy tools to guide financial growth





































